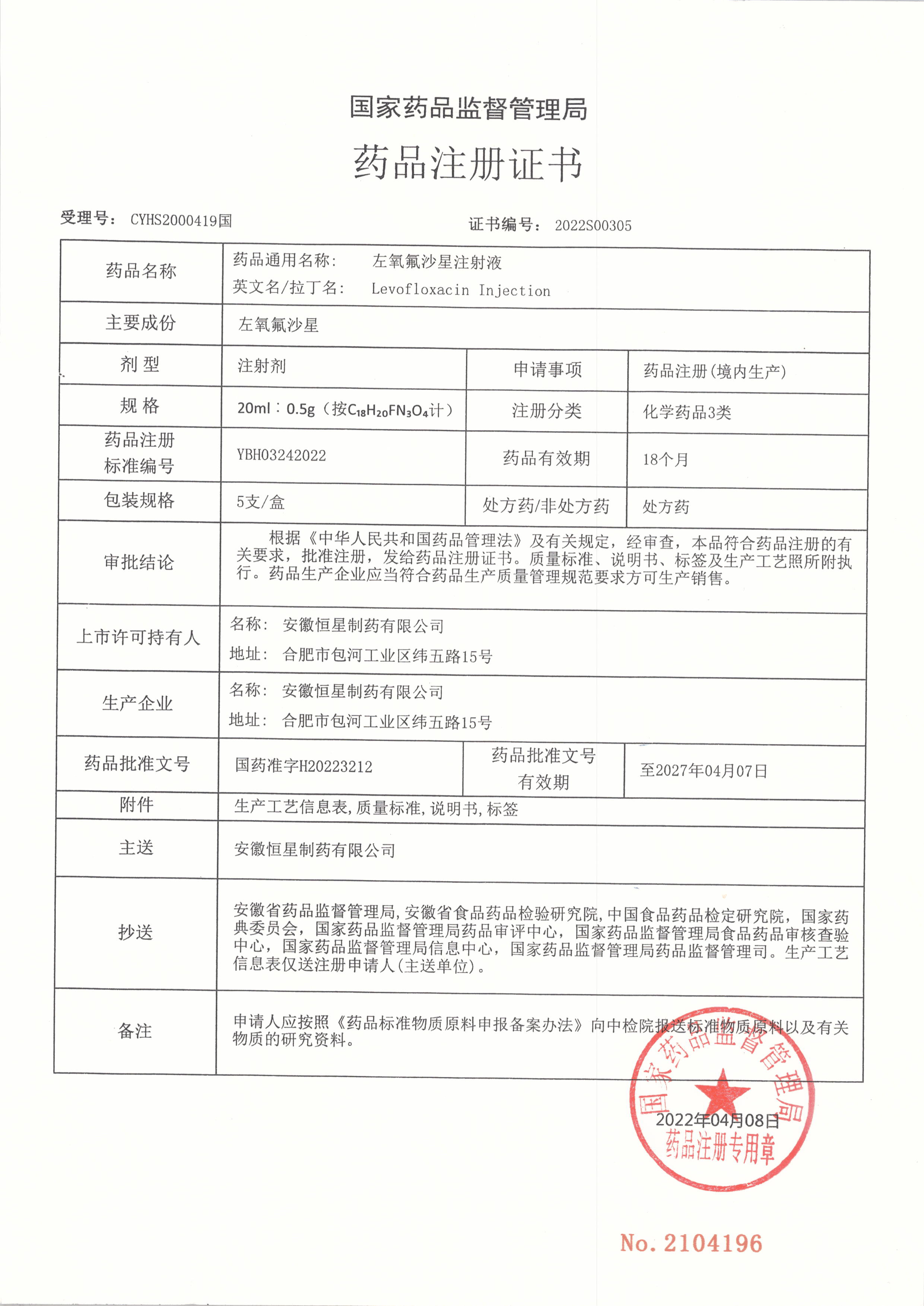
On April 20th, Anhui Xingxing Pharmaceutical Co., Ltd., a wholly-owned subsidiary of Huaren Pharmaceutical Co., Ltd. (300110), received the registration certificate for Class 3 levofloxacin injection of chemical drugs approved and issued by the National Drug Administration, with approval number H20223212 and validity until April 7, 2027.
Levofloxacin injection is mainly used to treat mild, moderate, and severe infections caused by sensitive strains of the following bacteria in adults (≥ 18 years old). This includes the following infections: hospital acquired pneumonia, community acquired pneumonia, acute bacterial sinusitis, acute bacterial attacks of chronic bronchitis, complex skin and skin structure infections, non complex skin and skin soft tissue infections, chronic bacterial prostatitis, complex urinary tract infections, acute pyelonephritis, non complex urinary tract infections, and inhalation anthrax (after exposure).
The original manufacturer of levofloxacin injection is Daiichi Mitsubishi Corporation of Japan, and Anhui Xingxing Pharmaceutical is the second enterprise in China to be approved under drug registration classification 3. According to the relevant provisions of the Announcement on the Evaluation of the Quality and Efficacy Consistency of Generic Drugs issued by the National Drug Administration (2017 No. 100), this product is deemed to have passed the evaluation of the quality and efficacy consistency of generic drugs. The approval of drug registration for levofloxacin injection this time will further enrich the company's product line, promote the construction and upgrading of product capabilities, and have a positive impact on the company's future development.

official account

MicroBlog